-
Notifications
You must be signed in to change notification settings - Fork 51
New issue
Have a question about this project? Sign up for a free GitHub account to open an issue and contact its maintainers and the community.
By clicking “Sign up for GitHub”, you agree to our terms of service and privacy statement. We’ll occasionally send you account related emails.
Already on GitHub? Sign in to your account
Discussions on simulating the worm's crawling by means of command neurons, motor neurons and muscle cells #82
Comments
|
Relevant: http://www.jneurosci.org/content/33/15/6380#sec-13 I'd suggest that these hypotheses can have tests built around them via c302 models to explore their implications. |
2) Proprioception in motor neurons is key for generating the forward locomotionEven when all the command interneurons were knocked out, C. elegans was able to generate the crawling [1]. This suggests the significant role of motor neurons in propagation of the bending waves. In general, rhythmic behavior is exhibited in animals thanks to the existence of neural circuits named as central pattern generator (CPG) [2]. There can be groups of CPG networks distributing rhythmic activities in an organism. CPG networks therefore should get coordinated with each other. Usually a sensory feedback mechanism exists for such coordination [3, 4, 5]. In C. elegans however, such sensory feedback does not exist due to lack of advanced sensory neurons. Accordingly, one argument suggests that the proprioceptive property can be "economically" exhibited by means of individual motor neurons [6, 8]. Electron microscopy illustrated that particularly cholinergic motor neurons (e.g. B-type), induce asynaptic processes all along the posterior without synaptic connections [6, 8]. Such mechanisms have been hypothesized as a proprioception process. Wen et al. in [8], conducted wonderful experiments on quantification of proprioception in the B-type motor neurons of C. elegans.
Figure 1. (Taken from [8]) Symbolic representation of connections from DB and VB motor neurons to some muscle cells through their neuromuscular junctions (Triangles) and their axons along the body of the worm. The asynaptic process shown in the figure illustrates the potential proprioceptive effect of the B-type motor neurons by means of their axons. Axons of the anterior DB motor neurons extend along the body of the worm to the posterior side making it possible to propagate a bending wave [8]. According to such findings, I believe for modeling the crawling of C. elegans, in our neuron or muscle model one should properly include the biomechanics of the undulatory movement in order to include the proprioception mechanisms. We will try this soon together with David! @lungd References: |
|
This recent article could be insightful for studying the proprioceptive coupling. A new computational method for a model of C. elegans biomechanics: Insights into elasticity and locomotion performance Netta Cohen, Thomas Ranner |
Sub-roadmap for the nervous system simulation
|

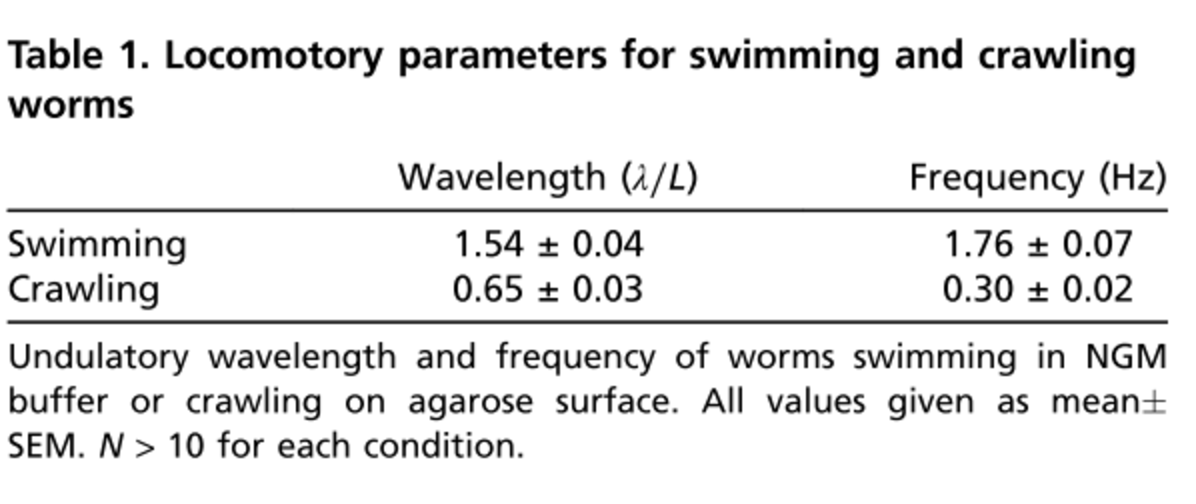
In a series of documents, I will discuss the role of the command-neurons, different types of motor neurons and their connectivity with the body-wall muscle cells. The ultimate goal is to derive a valid assumption on how the crawling happens in C. elegans and accordingly, simulate the response of the worm to a tap or touch stimulus in C302 and Sibernetics. My justifications will be based on the connectome dataset provided for the adult hermaphrodite by the WormWiring [1]. I will be investigating various neural circuits consisting of command neurons such as AVB and AVA, together with B-type, A-type, D-type and AS motor neurons including their connectivity to the muscles. The first discussion will be on a neural circuit comprised of AVB and B-type motor neurons. I will then discuss D-type and AS motor neurons within the next discussion. Afterwards, I will include the body-wall muscles into the neural circuit and discuss in details the propagation of an AVB-excitation into the motor neurons and muscle cells. I will finally repeat such analyses with AVA command neuron and A-type motor neurons and explain the neural circuit's architecture.
We then simulate our hypotheses in C302 and perform a parameter optimization based on our findings.
1) On the importance of the command neuron AVB and B-type motor-neurons in the worm’s crawling:
AVB command neuron together with B-type motor neurons function in the deriving the forward locomotion. There are seven dorsal and eleven ventral B-type motor neurons spread over the body of the worm. Considering a neural circuit, shown in Figure 1A and 1B, consist of only AVBL/R and all the B-type motor-neurons on the dorsal and ventral sides, one can highlight some attractive fundamental architectural design properties within the circuit:
Figure 1. AVB and B-type motor neurons neural circuit. A) AVBR\L and dorsal motor neuron neural circuit. Green lines are bidirectional gap junctions. Blue arrows represent excitatory synapses. Numbers on each line/arrow represent the number of synaptic connection between neurons. (Note that for instance 4/3, stands for 4 connections from AVBL and 3 connections from AVBR.) B) AVBR\L and ventral motor neuron neural circuit. C) Worm's muscles [2].
By looking at the sequential lateral connections among dorsal B-type motor-neurons, one can observe that DB1, DB2 and DB3 are strongly coupled through their gap-junctions while weakening their correlation rate with the rest of the synced motor-neurons DB4, DB5, DB6 and DB7, through a weak gap-junction between DB3 and DB4. This can potentially indicate that the activity of the dorsal B-type motor-neurons can be decoupled where the neurons 1 to 3 can be responsible for exciting part-A muscle cells shown in figure 1C, and the rest being responsible for the stimulation of the muscles depicted in part-B.
Such property holds for the ventral B-type motor neurons as well. Neurons VB2 and VB3 are strongly coupled while they get gradually decoupled to the rest of the motor neurons by means of the weak connections among VB3, VB4 and VB5. Therefore, we assume that the first group including VB1 to VB4 excites part-A muscles cells and second group including VB5 to VB11 excites part-B of the body-wall muscles.
Hypothesis 1: Like many other biological optimal networks such as beta cell hubs in islet functional architecture [3], I assume there exist hub neurons within the network of motor neurons in the C. elegans connectome which distribute commands within the network. This is explainable by looking at the connections from AVB to the motor neurons where the first group of motor neurons and specially their hubs (DB1 and VB2) are getting stimulated the most and in the next group DB7 and VB11 gets the most number of connections. The hypothesis can be further supported by an analysis on the connectivity of the motor neurons and muscle cells which will be included within the next discussions.
Such property has been observed also within the D-type dorsal and ventral motor neurons which will be explained in the next discussion.
References:
[1] wormwiring, http://wormwiring.org/hermaphrodite/herm.php
[2] wormbrowser, http://browser.openworm.org
[3] Johnston, Natalie R., et al. "Beta cell hubs dictate pancreatic islet responses to glucose." Cell Metabolism 24.3 (2016): 389-401.
The text was updated successfully, but these errors were encountered: